
Chemistry, 25.04.2020 23:13 samantha9430
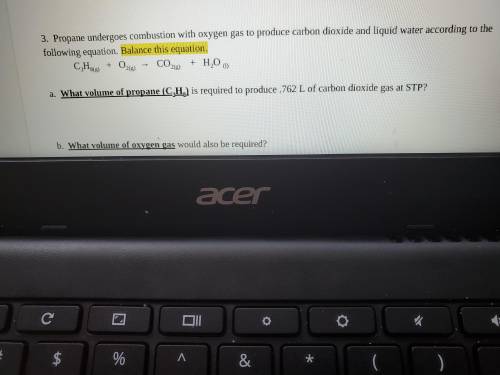
Propane undergoes combustion with oxygen gas to produce carbon dioxide and liquid water according to the following equation. Balance this equation.
C3H8(g) + O2(g) → CO2(g) + H2O (l)
a. What volume of propane (C3H8) is required to produce .762 L of carbon dioxide gas at STP?
b. What volume of oxygen gas would also be required?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
Propane undergoes combustion with oxygen gas to produce carbon dioxide and liquid water according to...
Questions



Arts, 16.11.2020 14:20

French, 16.11.2020 14:20

Mathematics, 16.11.2020 14:20

Mathematics, 16.11.2020 14:20



Mathematics, 16.11.2020 14:20

Mathematics, 16.11.2020 14:20





Mathematics, 16.11.2020 14:20





German, 16.11.2020 14:20



