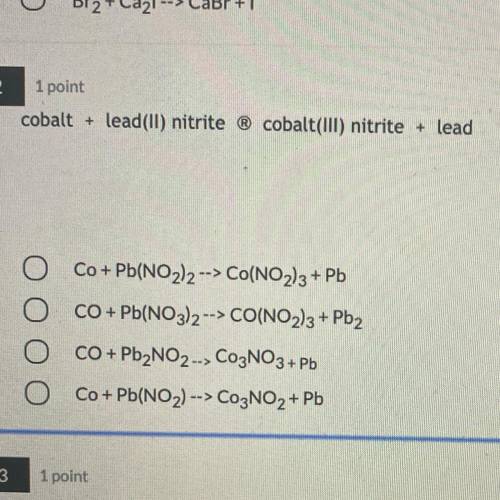
Cobalt + lead(II) nitrite ® cobalt(III) nitrite + lead
...

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
Questions

Mathematics, 09.12.2020 20:50


Mathematics, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50

Computers and Technology, 09.12.2020 20:50

Health, 09.12.2020 20:50



English, 09.12.2020 20:50


Physics, 09.12.2020 20:50

Mathematics, 09.12.2020 20:50




Social Studies, 09.12.2020 20:50



History, 09.12.2020 20:50