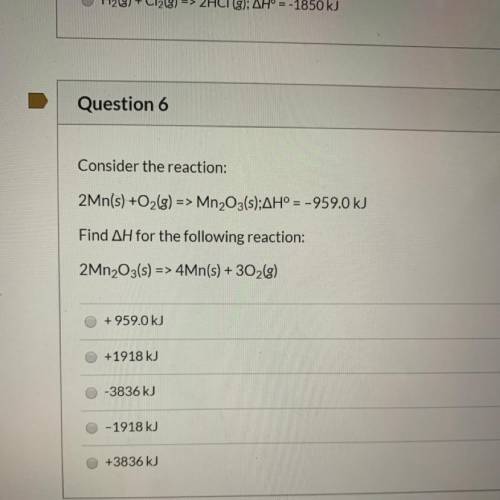
Consider the reaction:
2Mn(s) +O2(g) => Mn2O3(s);AH° = -959.0 kJ
Find AH for the foll...

Chemistry, 27.04.2020 01:29 CatsandDogsaredabest
Consider the reaction:
2Mn(s) +O2(g) => Mn2O3(s);AH° = -959.0 kJ
Find AH for the following reaction:
2Mn2O3(s) => 4Mn(s) + 302(g)
+959.0 kJ
+1918 kJ
-3836 kJ
O-1918 kJ
+3836 kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

You know the right answer?
Questions



Spanish, 28.09.2020 05:01

Social Studies, 28.09.2020 05:01




History, 28.09.2020 05:01

Biology, 28.09.2020 05:01

Computers and Technology, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01


History, 28.09.2020 05:01



Mathematics, 28.09.2020 05:01



