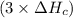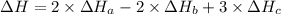2 Fe(s) + 6 HCl(aq) →2 FeCl3(aq) + 3 H2(g) ΔHa Fe2O3(s) + 6 HCl(aq) → 2 FeCI3(aq) + 3 H2O(l) ΔHb 2 H2(g) + O2(g) → 2 H2O(l) ΔHc Show how these equations must be summed together according to Hess's Law to determine ΔH for the combustion of iron (target equation shown below). Also show clearly how the ΔH values of each of the three reactions must be manipulated to determine the enthalpy of combustion of iron. 4 Fe(s) + 3 O2(g) → 2 Fe2O3(s) ΔH = ?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
2 Fe(s) + 6 HCl(aq) →2 FeCl3(aq) + 3 H2(g) ΔHa Fe2O3(s) + 6 HCl(aq) → 2 FeCI3(aq) + 3 H2O(l) ΔHb 2 H...
Questions


Mathematics, 10.03.2020 00:38

Mathematics, 10.03.2020 00:38

Health, 10.03.2020 00:38



Mathematics, 10.03.2020 00:38

Mathematics, 10.03.2020 00:38


History, 10.03.2020 00:38


Social Studies, 10.03.2020 00:38

Arts, 10.03.2020 00:38






Health, 10.03.2020 00:38

English, 10.03.2020 00:38

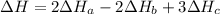
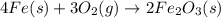
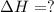
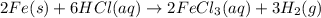 ;
; 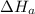
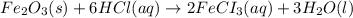 ;
; 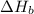
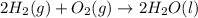 ;
; 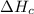
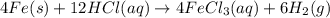 ;
; 
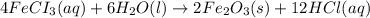 ;
; 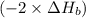
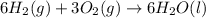 ;
;