
Suppose a 250.mL flask is filled with 0.70mol of H2 and 1.6mol of HCl. The following reaction becomes possible:
H2 (g) + Cl2 (g) <---> 2HCl (g)
The equilibrium constant K for this reaction is 0.262 at the temperature of the flask. Calculate the equilibrium molarity of HCl. Round your answer to two decimal places.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Suppose a 250.mL flask is filled with 0.70mol of H2 and 1.6mol of HCl. The following reaction become...
Questions

Chemistry, 25.08.2020 22:01




Advanced Placement (AP), 25.08.2020 22:01

Chemistry, 25.08.2020 22:01

Mathematics, 25.08.2020 22:01




History, 25.08.2020 22:01

Mathematics, 25.08.2020 22:01

Business, 25.08.2020 22:01

Mathematics, 25.08.2020 22:01






![\frac{[HCl]^2}{[H_{2}][Cl_{2}]}](/tpl/images/0648/3167/ae53b.png) = 0.262
= 0.262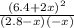 x = 0.285
x = 0.285 

