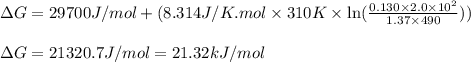Consider the malate dehydrogenase reaction from the citric acid cycle. Given the listed concentrations, calculate the free energy change for this reaction at energy change for this reaction at 37.0 ∘C37.0 ∘C (310 K). ΔG∘′ΔG∘′ for the reaction is +29.7 kJ/mol+29.7 kJ/mol . Assume that the reaction occurs at pH 7. [malate]=1.37 mM [malate]=1.37 mM [oxaloacetate]=0.130 mM [oxaloacetate]=0.130 mM [NAD+]=490 mM [NAD+]=490 mM [NADH]=2.0×102 mM

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3


Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Consider the malate dehydrogenase reaction from the citric acid cycle. Given the listed concentratio...
Questions


History, 15.04.2020 06:56


Biology, 15.04.2020 06:56



English, 15.04.2020 06:56



Mathematics, 15.04.2020 06:56

Biology, 15.04.2020 06:56


Mathematics, 15.04.2020 06:56


Health, 15.04.2020 06:56

Mathematics, 15.04.2020 06:56

History, 15.04.2020 06:57



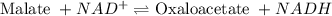
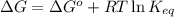
 = free energy of the reaction
= free energy of the reaction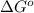 = standard Gibbs free energy = 29.7 kJ/mol = 29700 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = 29.7 kJ/mol = 29700 J/mol (Conversion factor: 1 kJ = 1000 J)![37^oC=[273+37]K=310K](/tpl/images/0648/3206/c6b28.png)
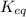 = Ratio of concentration of products and reactants =
= Ratio of concentration of products and reactants = ![\frac{\text{[Oxaloacetate]}[NADH]}{\text{[Malate]}[NAD^+]}](/tpl/images/0648/3206/4665e.png)
![\text{[Oxaloacetate]}=0.130mM](/tpl/images/0648/3206/da09e.png)
![[NADH]=2.0\times 10^2mM](/tpl/images/0648/3206/7312b.png)
![\text{[Malate]}=1.37mM](/tpl/images/0648/3206/1f6d8.png)
![[NAD^+]=490mM](/tpl/images/0648/3206/6a7be.png)