Chemistry, 06.05.2020 07:02 kaliloabousjbf
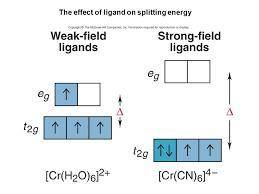
For the following give (1) oxidation # of metal, (2) number of d electrons, draw valence bond description of the complex, fill in metal and ligand valence electrons, give (3) metal orbitals that are hybridized, (4) type of hybridization, (5) molecular geometry, and (6) draw the crystal field de- scription of the octahedral complexes for a), c), and e).
[Cr(H2O)6]2+ (High spin)
(1) ___ (2) (3) (4) (5)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
For the following give (1) oxidation # of metal, (2) number of d electrons, draw valence bond descri...
Questions






Mathematics, 18.10.2020 07:01

Social Studies, 18.10.2020 07:01





Physics, 18.10.2020 07:01



English, 18.10.2020 07:01

Mathematics, 18.10.2020 07:01



Computers and Technology, 18.10.2020 07:01