
Chemistry, 06.05.2020 07:07 zitterkoph
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of nitrogen monoxide produced by the reaction of 0.050mol of ammonia . Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapo...
Questions


Chemistry, 27.06.2019 21:00




Biology, 27.06.2019 21:00






Social Studies, 27.06.2019 21:00






Mathematics, 27.06.2019 21:00

Mathematics, 27.06.2019 21:00

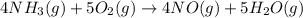
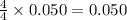 moles of nitrogen monoxide
moles of nitrogen monoxide

