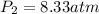Chemistry, 06.05.2020 07:10 tannercarr7428
Shaking up a soda bottles increase pressure by adding the number of gas particles (moles of carbon dioxide) into the inflexible can. If a soda bottle has an initial pressure of 2.50 atm and 0.150mol of CO2, then is shaken up so there are now 0.500 moles of carbon dioxide, what will the new pressure of the soda bottle be?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Shaking up a soda bottles increase pressure by adding the number of gas particles (moles of carbon d...
Questions


Mathematics, 23.08.2019 11:30

Mathematics, 23.08.2019 11:30

Biology, 23.08.2019 11:30

Computers and Technology, 23.08.2019 11:30

History, 23.08.2019 11:30



Mathematics, 23.08.2019 11:50


Mathematics, 23.08.2019 11:50




Mathematics, 23.08.2019 11:50

Geography, 23.08.2019 11:50




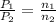
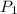 = initial pressure of gas = 2.50 atm
= initial pressure of gas = 2.50 atm = final pressure of gas = ?
= final pressure of gas = ?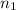 = initial number of moles of gas = 0.150 moles
= initial number of moles of gas = 0.150 moles = final number of moles of gas = 0.500 mol
= final number of moles of gas = 0.500 mol