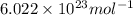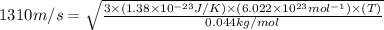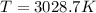Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal to 44.0 g/mol ) in a flame is found to be 1310 m/s. What temperature T does this represent? The Boltzmann constant is k=1.38×10−23 J/K and Avogadro's number is A=6.022×1023 mol−1.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal...
Questions






Mathematics, 23.03.2021 06:50

Mathematics, 23.03.2021 06:50

Mathematics, 23.03.2021 06:50




Biology, 23.03.2021 06:50

English, 23.03.2021 06:50

Mathematics, 23.03.2021 06:50


Mathematics, 23.03.2021 06:50




Mathematics, 23.03.2021 06:50

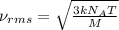
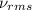 = root mean square speed = 1310 m/s
= root mean square speed = 1310 m/s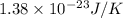
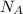 = Avogadro’s number =
= Avogadro’s number =