
Chemistry, 06.05.2020 07:23 davelopez979
A chemist measures the energy change Delta H during the following
2Fe2O3(s) > FeO(s) + O2(g). ΔH = 560 kJ
1) This reactions is .
O Endothermic
O Exothermic
2) Suppose 94.2g of Fe2O3 react. will any heat be relased or absorbed?
O Yes, absorbed.
O Yes, released.
O No.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
A chemist measures the energy change Delta H during the following
2Fe2O3(s) > FeO(s) + O2(g...
2Fe2O3(s) > FeO(s) + O2(g...
Questions







Mathematics, 05.11.2021 04:20





Mathematics, 05.11.2021 04:20






Mathematics, 05.11.2021 04:30


Physics, 05.11.2021 04:30

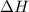 for the reaction comes out to be positive.
for the reaction comes out to be positive.

