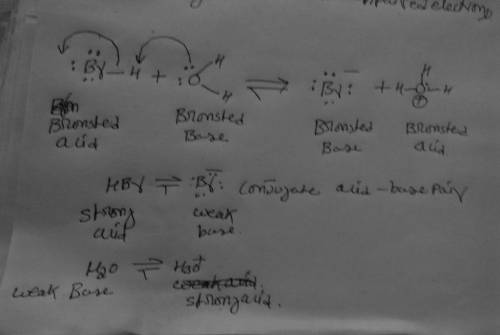
The wet cotton traps any HBr that escapes the reaction mixture. The trapping reaction is simply what happens when HBr dissolves in water, but it is still an acid/base reaction that is in principle reversible. Complete the acid/base equilibrium started for you below, draw the curved arrow pushing in BOTH directions and identify the stronger and weaker Bronsted acid and base on each side of the equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1
You know the right answer?
The wet cotton traps any HBr that escapes the reaction mixture. The trapping reaction is simply what...
Questions

Computers and Technology, 27.08.2019 14:30

Social Studies, 27.08.2019 14:30

Mathematics, 27.08.2019 14:30

Mathematics, 27.08.2019 14:30





Mathematics, 27.08.2019 14:50

History, 27.08.2019 14:50


Biology, 27.08.2019 14:50

Social Studies, 27.08.2019 14:50

Physics, 27.08.2019 14:50



History, 27.08.2019 14:50

English, 27.08.2019 14:50


Chemistry, 27.08.2019 14:50