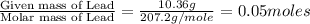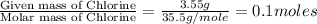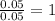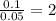Chemistry, 06.05.2020 07:29 jalexyinez
In an experiment similar to the zinc chloride experiment, a student placed a piece of lead in hydrochloric acid. Hydrogen gas was given off, and then the liquid was boiled off. The remaining solid, lead chloride was massed. Use the data below to determine the empirical formula of lead chloride.
mass of beaker 204.35 g
mass of lead and beaker before reaction 214.71 g
mass of lead chloride and beaker after reaction 218.26 g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

You know the right answer?
In an experiment similar to the zinc chloride experiment, a student placed a piece of lead in hydroc...
Questions


Health, 27.01.2020 17:31



Mathematics, 27.01.2020 17:31

Mathematics, 27.01.2020 17:31

English, 27.01.2020 17:31



Mathematics, 27.01.2020 17:31

History, 27.01.2020 17:31

Chemistry, 27.01.2020 17:31

Biology, 27.01.2020 17:31



Mathematics, 27.01.2020 17:31

Chemistry, 27.01.2020 17:31

Chemistry, 27.01.2020 17:31


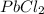
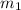 = 204.35 g
= 204.35 g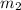 = 214.71 g
= 214.71 g = 218.26 g
= 218.26 g = [214.71 - 204.35] = 10.36 g
= [214.71 - 204.35] = 10.36 g = [218.26 - 214.71] = 3.55 g
= [218.26 - 214.71] = 3.55 g