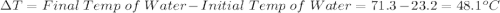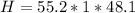Chemistry, 06.05.2020 06:05 fdougie111
You are running a calorimetry experiment where you are trying to determine the number of Calories (with a capital C!) in a peanut. You set up your aluminum can of water and take all your initial data, putting it in the table below. Then, you set your peanut ON FIRE You finish filling out your table once the peanut has gone out. How many Calories of heat did your peanut release? Round your answer to two digits after the decimal point.
Initial Mass of Peanut 3.11 grams
Final Mass of Peanut 0.52 grams
Mass of Water 55.2 grams
Initial Temp of Water 23.2 degrees C
Final Temp of Water 71.3 degrees C

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
You know the right answer?
You are running a calorimetry experiment where you are trying to determine the number of Calories (w...
Questions





Mathematics, 13.11.2019 02:31

Health, 13.11.2019 02:31

Mathematics, 13.11.2019 02:31

Mathematics, 13.11.2019 02:31

History, 13.11.2019 02:31

History, 13.11.2019 02:31

English, 13.11.2019 02:31

Mathematics, 13.11.2019 02:31

Computers and Technology, 13.11.2019 02:31

History, 13.11.2019 02:31

Mathematics, 13.11.2019 02:31

Mathematics, 13.11.2019 02:31


Mathematics, 13.11.2019 02:31

Mathematics, 13.11.2019 02:31

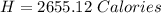
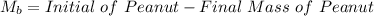
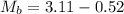
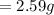
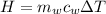
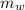 is the mass of water which is given as
is the mass of water which is given as 
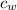 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of 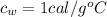
 is the change in temperature which can be evaluated as follows
is the change in temperature which can be evaluated as follows