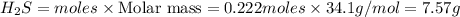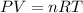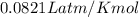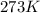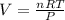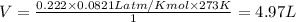Chemistry, 06.05.2020 06:12 harleyandpope90
If 25.0 grams of Sb2S3 reacts with an excess of hydrochloric acid, how many grams of H2S are formed? What volume does the H2S formed occupy under conditions of STP?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
If 25.0 grams of Sb2S3 reacts with an excess of hydrochloric acid, how many grams of H2S are formed?...
Questions



History, 03.02.2021 22:40



Physics, 03.02.2021 22:40

Physics, 03.02.2021 22:40

Arts, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40


World Languages, 03.02.2021 22:40

English, 03.02.2021 22:40

History, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40


English, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40


History, 03.02.2021 22:40

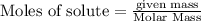
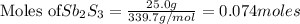
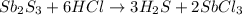
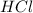 is the excess reagent,
is the excess reagent, 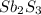 is the limiting reagent and it limits the formation of product.
is the limiting reagent and it limits the formation of product.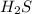
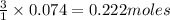 of
of