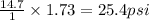Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1


You know the right answer?
What is the pressure of the air in a car tire (in psi) if it is 1.73 atm?...
Questions


Mathematics, 15.01.2021 05:00

Mathematics, 15.01.2021 05:00



Mathematics, 15.01.2021 05:00

Arts, 15.01.2021 05:00



Biology, 15.01.2021 05:00


Mathematics, 15.01.2021 05:00


Social Studies, 15.01.2021 05:00


Mathematics, 15.01.2021 05:00


English, 15.01.2021 05:00

Mathematics, 15.01.2021 05:00

History, 15.01.2021 05:00