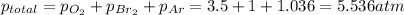Chemistry, 06.05.2020 06:32 nbunny7208
A metal tank contains three gases: oxygen, argon, and bromine. If the partial pressures of the three gases in the tank are 3.5 atm of O2, 760.0 mmHg of
Br2,and 105 kPa of Ar, what is the total pressure inside of the tank in atmospheres?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
A metal tank contains three gases: oxygen, argon, and bromine. If the partial pressures of the three...
Questions

Engineering, 14.12.2020 08:00



Business, 14.12.2020 08:00

History, 14.12.2020 08:00


English, 14.12.2020 08:00

Biology, 14.12.2020 08:00

Mathematics, 14.12.2020 08:00

Business, 14.12.2020 08:00

Mathematics, 14.12.2020 08:00

History, 14.12.2020 08:00



Mathematics, 14.12.2020 08:00



Mathematics, 14.12.2020 08:10


Mathematics, 14.12.2020 08:10