
Chemistry, 15.10.2019 18:40 dylankrenek
Butane, c4h10, reacts with oxygen, o2, to form water, h2o, and carbon dioxide, co2, as shown in the following chemical equation: 2c4h10(g)+13o2(g)-> 10h2o(g)+8co2(g)
calculate the mass of water produced when 1.77 grams of butane reacts with excessive oxygen?
calculate the mass of butane needed to produce 71.6 of carbon dioxide.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Butane, c4h10, reacts with oxygen, o2, to form water, h2o, and carbon dioxide, co2, as shown in the...
Questions



Social Studies, 13.02.2021 02:00

Mathematics, 13.02.2021 02:00


Biology, 13.02.2021 02:00

Social Studies, 13.02.2021 02:00

English, 13.02.2021 02:00

Mathematics, 13.02.2021 02:00

Mathematics, 13.02.2021 02:00




Mathematics, 13.02.2021 02:00

Biology, 13.02.2021 02:00


English, 13.02.2021 02:10


Mathematics, 13.02.2021 02:10

English, 13.02.2021 02:10

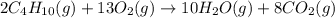
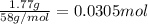
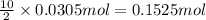 of water
of water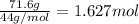
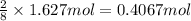 of butane
of butane

