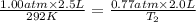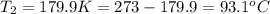Chemistry, 06.05.2020 04:58 scadengo123
Mr. Fredrickson fills 1 million balloons to a volume of 2.5 liters each at sea level (1.00 atm), at 19 degrees Celsius. If the balloons carry his house to a height of 20,000 feet (0.77 atm), what is the temperature (Celsius) if they each shrink to a volume of 2.0 liters?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
Mr. Fredrickson fills 1 million balloons to a volume of 2.5 liters each at sea level (1.00 atm), at...
Questions












Computers and Technology, 21.06.2019 22:50

English, 21.06.2019 22:50




History, 21.06.2019 22:50

Mathematics, 21.06.2019 22:50

Mathematics, 21.06.2019 22:50

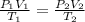
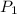 = initial pressure of gas = 1.00 atm
= initial pressure of gas = 1.00 atm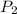 = final pressure of gas = 0.77 atm
= final pressure of gas = 0.77 atm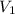 = initial volume of gas = 2.5 L
= initial volume of gas = 2.5 L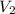 = final volume of gas = 2.0 L
= final volume of gas = 2.0 L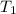 = initial temperature of gas =
= initial temperature of gas = 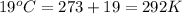
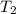 = final temperature of gas = ?
= final temperature of gas = ?