
Chemistry, 06.05.2020 03:59 michael1498
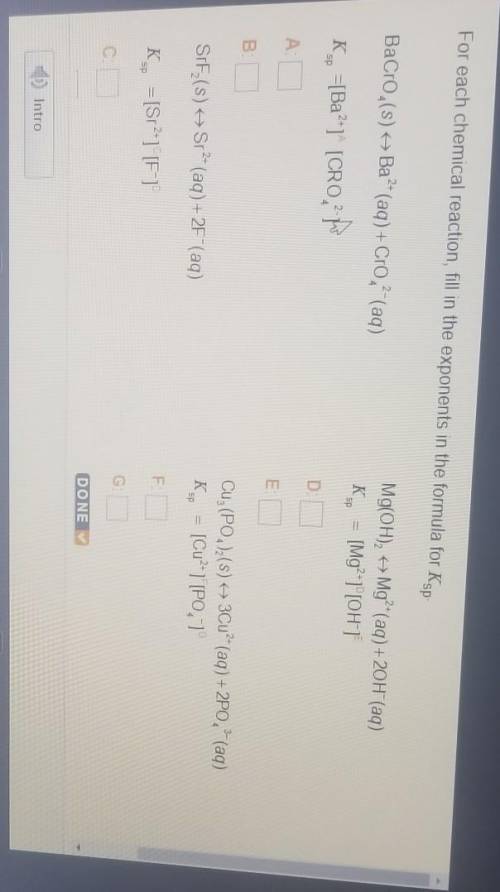
For each chemical reaction, fill in the exponents in the formula for Ks
BaCro (s) + Ba?- (aq) + Cro?- (aq)
K., =[Ba?"1" [CRO-
Mg(OH)2 + Mg²+(aq) + 20H (aq)
Ks = [Mg²+1"[OH]
SIF (S)
Sr- (aq) + 2F"(aq)
Cuz (PO4)2(s) + 3Cu?- (aq) + 2PO, 2 (aq)
Kg = [Cu²+ ][PO, 1
K., = [Sr2-1 [F-1

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
For each chemical reaction, fill in the exponents in the formula for Ks
BaCro (s) + Ba?- (aq) +...
BaCro (s) + Ba?- (aq) +...
Questions







Business, 03.02.2022 01:00

Mathematics, 03.02.2022 01:00

Mathematics, 03.02.2022 01:00

SAT, 03.02.2022 01:00

Social Studies, 03.02.2022 01:00

Biology, 03.02.2022 01:00






Mathematics, 03.02.2022 01:00

Mathematics, 03.02.2022 01:00



