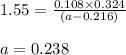Chemistry, 06.05.2020 04:03 Benjamincompton07
The equilibrium constant, Kc, for the following reaction is 1.55 at 667 K.
2NH3(g) <> N2(g) + 3H2(g)
When a sufficiently large sample of NH3(g) is introduced into an evacuated vessel at 667 K, the equilibrium concentration of H2(g) is found to be 0.324 M.
Calculate the concentration of NH3 in the equilibrium mixture.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.55 at 667 K.
2NH3(g) <...
2NH3(g) <...
Questions




Computers and Technology, 12.11.2019 00:31








Mathematics, 12.11.2019 00:31








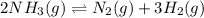
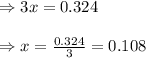
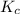 for above equation follows:
for above equation follows:![K_c=\frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0646/0178/a64ad.png)