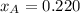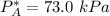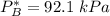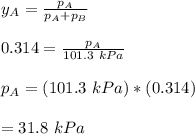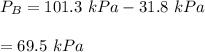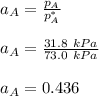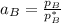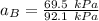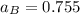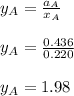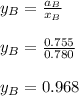Chemistry, 06.05.2020 03:07 rachel2005smith
By measuring the equilibrium between liquid and vapor phases of a binary solution at 30°C at 1atm, it was found that xA=0.220 when yA=0.314. Calculate the activities and activity coefficients of both components in the solution. The vapor pressures of the pure components at this temperature are 73kPa and 92.1kPa.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:50
What type of reaction is illustrated? 2c12o5 = 2cl2 + 502
Answers: 2

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1
You know the right answer?
By measuring the equilibrium between liquid and vapor phases of a binary solution at 30°C at 1atm, i...
Questions

Social Studies, 29.10.2020 16:10






English, 29.10.2020 16:10

Mathematics, 29.10.2020 16:10

English, 29.10.2020 16:10




Health, 29.10.2020 16:10


Biology, 29.10.2020 16:10





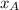 is the mole fraction in the liquid
is the mole fraction in the liquid 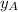 is the mole fraction in the vapor
is the mole fraction in the vapor