
Chemistry, 06.05.2020 03:11 acavalieri72
Predict whether ΔS for each reaction would be greater than zero, less than zero, or too close to zero to decide.
ΔS > 0; ΔS < 0; too close to decide
I2(g) + Cl2(g) > 2ICl(g)
2NOBr(g) > 2NO(g) + Br2(g)
CO2(g) + H2(g) > CO(g) + H2O(g)
2H2O2(I) > 2H2O(I) + O2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Predict whether ΔS for each reaction would be greater than zero, less than zero, or too close to zer...
Questions



Mathematics, 23.03.2021 18:40

Mathematics, 23.03.2021 18:40






Mathematics, 23.03.2021 18:40


Mathematics, 23.03.2021 18:40

Social Studies, 23.03.2021 18:40


English, 23.03.2021 18:40



Mathematics, 23.03.2021 18:40

Mathematics, 23.03.2021 18:40

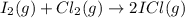 : too close to decide.
: too close to decide.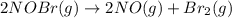 :
: 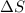 > 0.
> 0.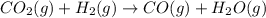 : too close to decide.
: too close to decide.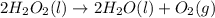 :
: 

