Chemistry, 06.05.2020 03:28 alwaysneedhelp84
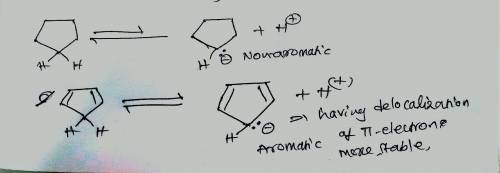
The pKapKa of cyclopentane is > 60, which is about what is expected for a hydrogen that is bonded to an sp3sp3 carbon. Explain why cyclopentadiene is a much stronger acid (pKapKa of 15), even though it too involves the loss of a proton from an sp3sp3 carbon. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3
You know the right answer?
The pKapKa of cyclopentane is > 60, which is about what is expected for a hydrogen that is bonded...
Questions


Mathematics, 07.01.2020 23:31

History, 07.01.2020 23:31






Mathematics, 07.01.2020 23:31

Mathematics, 07.01.2020 23:31

Arts, 07.01.2020 23:31





Mathematics, 07.01.2020 23:31