
Methanol, is made by using a catalyst to react carbon monoxide with hydrogen at high temperature and pressure. Assuming that 450.0 of CO and 825 of H2O are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
Which reactant is in excess?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 13:30
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 1
You know the right answer?
Methanol, is made by using a catalyst to react carbon monoxide with hydrogen at high temperature and...
Questions


Mathematics, 30.10.2020 02:00


Mathematics, 30.10.2020 02:00


Mathematics, 30.10.2020 02:00


Biology, 30.10.2020 02:00


History, 30.10.2020 02:00


Mathematics, 30.10.2020 02:00

Biology, 30.10.2020 02:00

Mathematics, 30.10.2020 02:00

Mathematics, 30.10.2020 02:00





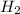 are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)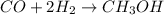
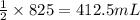 of carbon monoxide
of carbon monoxide

