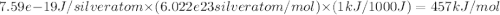The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface. (a) Is it easier to remove an electron from a gaseous silver atom or from the surface of solid silver (ϕ = 7.59×10−19 J; IE = 731 kJ/mol)? (b) Explain the results in terms of the electron-sea model of metallic bonding.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3
You know the right answer?
The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface...
Questions

History, 28.06.2019 00:30

Computers and Technology, 28.06.2019 00:30

History, 28.06.2019 00:30

Chemistry, 28.06.2019 00:30





Computers and Technology, 28.06.2019 00:30


Computers and Technology, 28.06.2019 00:30