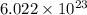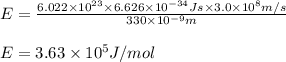Chemistry, 06.05.2020 02:01 mildred3645
An emission line of sodium has a wavelength of 330 nm. Calculate the energy of a photon of light emitted in J/ atom, and the energy emitted per mole of Na atoms at this wavelength.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
An emission line of sodium has a wavelength of 330 nm. Calculate the energy of a photon of light emi...
Questions





Mathematics, 28.10.2019 10:31

English, 28.10.2019 10:31

Biology, 28.10.2019 10:31


Computers and Technology, 28.10.2019 10:31



Mathematics, 28.10.2019 10:31





Biology, 28.10.2019 10:31

Mathematics, 28.10.2019 10:31


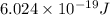 and energy emitted per mole of sodium atoms is
and energy emitted per mole of sodium atoms is 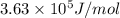
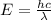
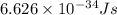
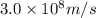
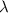 = wavelength of photon = 330 nm =
= wavelength of photon = 330 nm = 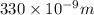 (Conversion factor:
(Conversion factor: 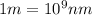 )
)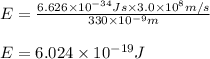
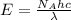
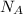 = Avogadro's number =
= Avogadro's number =