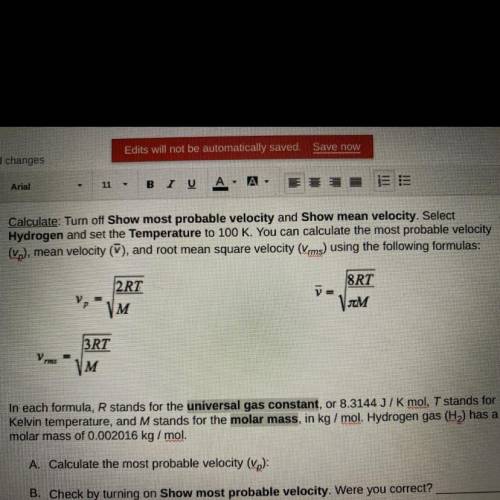
In each formula, R stands for the universal gas constant, or 8.3144 J/K mol, T stands for
Kelv...

Chemistry, 06.05.2020 02:14 vandarughb5653
In each formula, R stands for the universal gas constant, or 8.3144 J/K mol, T stands for
Kelvin temperature, and M stands for the molar mass, in kg/mol. Hydrogen gas (H) has a
molar mass of 0.002016 kg/mol.
A. Calculate the most probable velocity (vp):
B. Check by turning on Show most probable velocity. Were you correct?
C. Calculate the mean velocity (V):
D. Check by turning on Show mean velocity. Were you correct?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1


Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
Questions


Mathematics, 12.03.2022 07:30


Mathematics, 12.03.2022 07:30


Mathematics, 12.03.2022 07:30



Biology, 12.03.2022 07:40

Mathematics, 12.03.2022 07:40


Mathematics, 12.03.2022 07:40


Mathematics, 12.03.2022 07:40



SAT, 12.03.2022 07:40





