
A chemist designs a galvanic cell that uses these two half-reactions:
O2 (g) + 4H+(aq) + 4e− → 2H2O (l) Eo =+1.23V
Zn+2 (aq) + 2e− → Zn(s) Eo=−0.763V
Answer the following questions about this cell.
Write a balanced equation for the half-reaction that happens at the cathode.
Write a balanced equation for the half-reaction that happens at the anode.
Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2



Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
A chemist designs a galvanic cell that uses these two half-reactions:
O2 (g) + 4H+(aq) +...
O2 (g) + 4H+(aq) +...
Questions







Biology, 28.10.2020 18:10

Mathematics, 28.10.2020 18:10






Physics, 28.10.2020 18:10






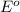 reduction potential will always get reduced and will undergo reduction reaction.
reduction potential will always get reduced and will undergo reduction reaction. 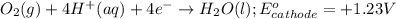
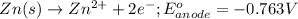 ( × 2)
( × 2)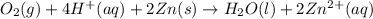
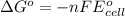
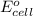 of the reaction, we use the equation:
of the reaction, we use the equation: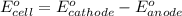
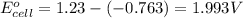
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Zn^{2+}]^2}{[H^{+}]^4\times p_{O_2}}](/tpl/images/0645/2279/2e91a.png)


