Chemistry, 06.05.2020 00:15 dpinzoner5952
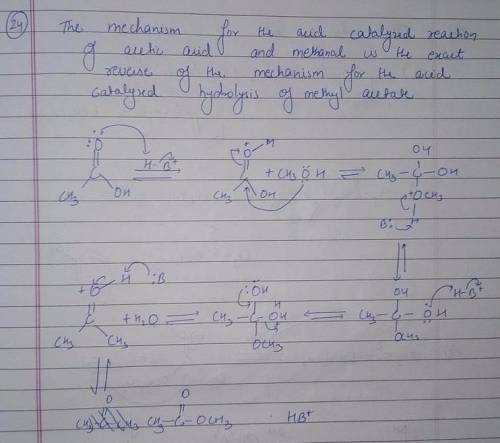
Using the mechanism for the acid-catalyzed hydrolysis of an ester as your guide, write the mechanism-showing all the curved arrows-for the acid-catalyzed reaction of acetic acid and methanol to form methyl acetate. All proton-donating and proton-removing species are given on canvas.
there are 7 boxesO
||
the first one has H3C-C-OH with OH+ --H
O
||
and the final product is H3C-C-O-CH3 with H3O+

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
Using the mechanism for the acid-catalyzed hydrolysis of an ester as your guide, write the mechanism...
Questions





History, 01.09.2020 21:01


Mathematics, 01.09.2020 21:01


English, 01.09.2020 21:01

Mathematics, 01.09.2020 21:01

History, 01.09.2020 21:01



History, 01.09.2020 21:01

Mathematics, 01.09.2020 21:01

History, 01.09.2020 21:01

History, 01.09.2020 21:01


Social Studies, 01.09.2020 21:01

Chemistry, 01.09.2020 21:01