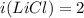Two aqueous solutions are prepared: 1.00 m Na2CO3 and 1.00 m LiCl. Which of the following statements is true?
A. The Na2CO3 solution has a higher osmotic pressure and higher vapor pressure than the LiCl solution.
B. The Na2CO3 solution has a higher osmotic pressure and higher boiling point than the LiCl solution.
C. The Na2CO3 solution has a lower osmotic pressure and lower vapor pressure than the LiCI solution.
D. The Na2CO3 solution has a lower osmotic pressure and higher boiling point than the LiCl solution.
E. None of these statements is true.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3



Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Two aqueous solutions are prepared: 1.00 m Na2CO3 and 1.00 m LiCl. Which of the following statements...
Questions

Mathematics, 07.05.2021 01:00


Law, 07.05.2021 01:00




Mathematics, 07.05.2021 01:00




Chemistry, 07.05.2021 01:00

English, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00


Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Chemistry, 07.05.2021 01:00

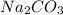 solution has a higher osmotic pressure and higher boiling point than LiCl solution.
solution has a higher osmotic pressure and higher boiling point than LiCl solution.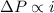 ,
, 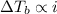 and
and 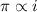
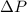 ,
, 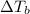 and
and  are lowering of vapor pressure, elevation in boiling point and osmotic pressure of solution respectively.
are lowering of vapor pressure, elevation in boiling point and osmotic pressure of solution respectively.  is van't hoff factor.
is van't hoff factor.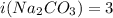 and
and