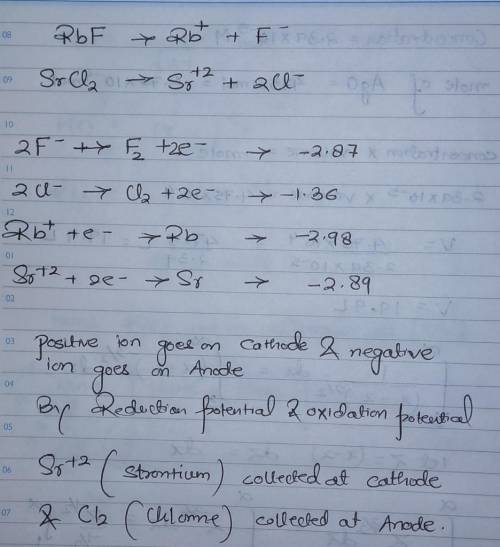Chemistry, 06.05.2020 00:22 osmanysalvador9
In the electrolysis of a molten mixture of RbF and SrCl2, identify the product that forms at the negative electrode and at the positive electrode. The cell temperature must be high enough to keep the salt mixture molten hence the metal appears as a liquid and the halogen as a gas. product at the negative electrode (cathode) product at the positive electrode (anode)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1


Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
In the electrolysis of a molten mixture of RbF and SrCl2, identify the product that forms at the neg...
Questions


Mathematics, 08.03.2021 18:00

Mathematics, 08.03.2021 18:00



Social Studies, 08.03.2021 18:00



Mathematics, 08.03.2021 18:00



Mathematics, 08.03.2021 18:00


Social Studies, 08.03.2021 18:00

Biology, 08.03.2021 18:00