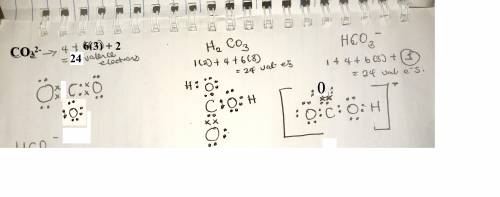Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Draw the lewis structure for CO2, H2CO3, HCO3-, and CO3 2-.Rank these in order of increasing attract...
Questions

Business, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Social Studies, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30


Mathematics, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30


Mathematics, 20.10.2019 15:30





English, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Business, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

Mathematics, 20.10.2019 15:30

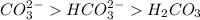
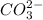 will accept protons more readily than the bicarbonate ion,
will accept protons more readily than the bicarbonate ion, 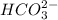 . Carbonic acid,
. Carbonic acid, 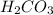 will not accept any more protons, hence it is the least attractive to water molecule, even though soluble.
will not accept any more protons, hence it is the least attractive to water molecule, even though soluble.