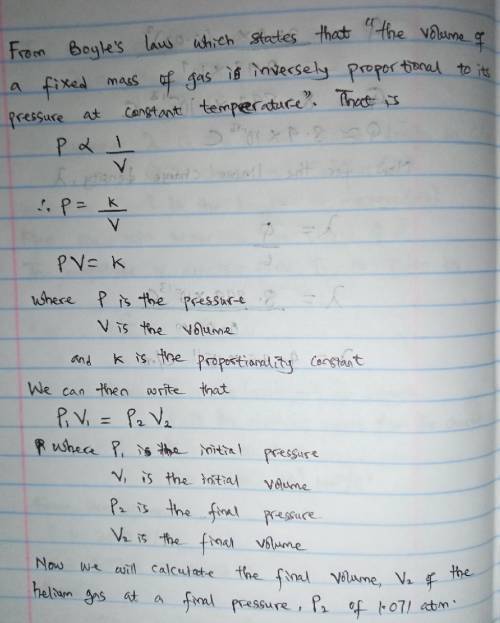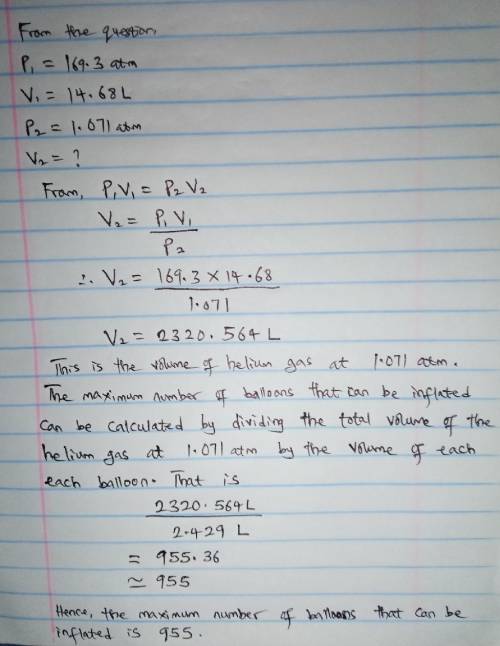Chemistry, 05.05.2020 22:21 mvazquez298
A cylinder containing 14.68 L of helium gas at a pressure of 169.3 atm is to be used to fill toy balloons to a pressure of 1.071 atm. Each inflated balloon has a volume of 2.429 L. What is the maximum number of balloons that can be inflated? Report your answer to 1 decimal place. (Remember that 14.68 L of helium at 1.071 atm will remain in the exhausted (empty) cylinder)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3


Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
A cylinder containing 14.68 L of helium gas at a pressure of 169.3 atm is to be used to fill toy bal...
Questions

Mathematics, 02.12.2019 22:31

Advanced Placement (AP), 02.12.2019 22:31

History, 02.12.2019 22:31

Geography, 02.12.2019 22:31

Mathematics, 02.12.2019 22:31






Health, 02.12.2019 22:31




Mathematics, 02.12.2019 22:31





Mathematics, 02.12.2019 22:31