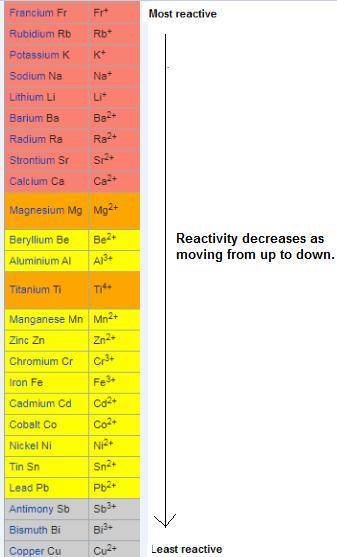
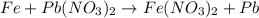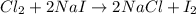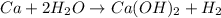"complete the equations for these single-replacement reactions in aqueous solutions. balance each equation. write "no reaction" if a reactions doesn't occur. use the activity series chart you copied into your notes."
a.) fe + pb(no3)2->
b.) cl2 + nai ->
c.) ca + h2o ->

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
"complete the equations for these single-replacement reactions in aqueous solutions. balance each eq...
Questions






Mathematics, 26.07.2019 10:30






Business, 26.07.2019 10:30


Biology, 26.07.2019 10:30





History, 26.07.2019 10:30