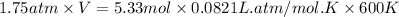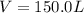Chemistry, 05.05.2020 21:22 estebencampos69
At 600 K and 1.75 atm, if 16.00 grams of H2 will reacts with excess N2 what volume in liters will be produced of NH3?
N2(g) + 3 H2(g) --> 2 NH3(g)
(R = 0.0821 L atm/mol K)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1
You know the right answer?
At 600 K and 1.75 atm, if 16.00 grams of H2 will reacts with excess N2 what volume in liters will be...
Questions








Geography, 28.07.2019 15:20






History, 28.07.2019 15:20

Social Studies, 28.07.2019 15:20





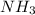 produced will be, 150.0 L
produced will be, 150.0 L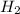
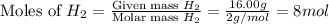
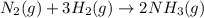
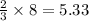 mole of
mole of