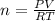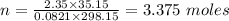Chemistry, 05.05.2020 21:21 janiyahmcgolley
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reaction:
CH4 (s) + 2 O2 (g) --> CO2 (g) + 2 H2O (l)
What mass of methane (in grams) will require 35.15 L of oxygen to fully react? The pressure is 2.35 atm and the temperature is 15 °C. (R = 0.0821 L atm/mol K)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reactio...
Questions

Biology, 16.10.2020 20:01



Mathematics, 16.10.2020 20:01

Engineering, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01





Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01




Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01


Geography, 16.10.2020 20:01