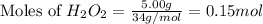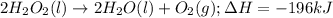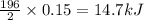Hydrogen peroxide can decompose to water and oxygen by the following reaction:
2h2o2 (l...

Chemistry, 31.01.2020 10:05 lauren21bunch
Hydrogen peroxide can decompose to water and oxygen by the following reaction:
2h2o2 (l) 2h2o(l) + o2(g) enthalpy= -196kj
calculate the value of q when 5.00g of h20(l) decomposes at constant pressure.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Questions

















Computers and Technology, 29.11.2019 06:31



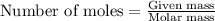
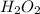 = 5.00 g
= 5.00 g