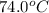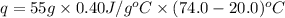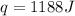Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1


Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
The specific heat of copper is 0.40 joules/ g °c. How much heat is needed in joules to change the te...
Questions

Biology, 01.09.2019 07:00


English, 01.09.2019 07:00


Mathematics, 01.09.2019 07:00

Health, 01.09.2019 07:00

Mathematics, 01.09.2019 07:00


Mathematics, 01.09.2019 07:00

Social Studies, 01.09.2019 07:00

History, 01.09.2019 07:00

Mathematics, 01.09.2019 07:00

Social Studies, 01.09.2019 07:00







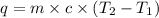
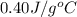
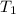 = initial temperature =
= initial temperature = 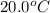
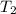 = final temperature =
= final temperature =