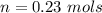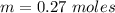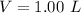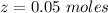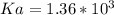Chemistry, 05.05.2020 17:06 jettskii214
A solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the present in the buffer solution. The Ka of nitrous acid is 1.36 × 10-3.A) H2OB) H3O+C) nitrite ionD) nitrous acidE) This is a buffer solution: the pH does not change upon addition of acid or base.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 11:50
Charles's law describes the relationship of the volume and temperature of gas at a constant mass and pressure. according to this law, what would happen to the temperature of the gas if its volume decreased from 1.0 l to 0.50 l?
Answers: 3

Chemistry, 23.06.2019 17:30
Consider the lewis structure for ch4 methane is this molecule polar or nonpolar
Answers: 1

Chemistry, 23.06.2019 21:00
Jenny takes a piece of paper and dipped it in a bowl of water the paper is now damp and mushy which of the following is our true about the change that occurs when the paper gets wet
Answers: 3
You know the right answer?
A solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in wate...
Questions


Physics, 27.09.2019 23:10


Biology, 27.09.2019 23:10

Mathematics, 27.09.2019 23:10






Law, 27.09.2019 23:10

Geography, 27.09.2019 23:10

History, 27.09.2019 23:10

Social Studies, 27.09.2019 23:10