Chemistry, 05.05.2020 16:09 alexabrandon1848
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M solution of HCl at 25°C. Enter your numbers to 2 decimal places. Kb = 4.4x10-4 What is the pH of the methylamine solution before titrant is added? 11.91 How many milliliters of titrant are required to reach the equivalence point? 36.59 What is the pH at the equivalence point?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M soluti...
Questions

Social Studies, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01












History, 12.08.2020 05:01


Computers and Technology, 12.08.2020 05:01




![pOH = \frac{1}{2}[pK_b \ - \ log \ C]](/tpl/images/0640/0162/873d4.png)
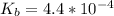
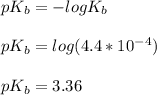
![pOH = \frac{1}{2}[3.36\ - \ log \0.15]](/tpl/images/0640/0162/71cf9.png)
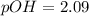
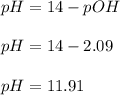
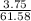
![pOH = \frac{1}{2}[pK_w+pK_b+log \ C]](/tpl/images/0640/0162/af00e.png)
![pOH = \frac{1}{2}[14+3.36+log \ 0.061]](/tpl/images/0640/0162/443df.png)
![pOH = \frac{1}{2}[16.15]](/tpl/images/0640/0162/bf604.png)