Chemistry, 05.05.2020 16:13 Ashtree519
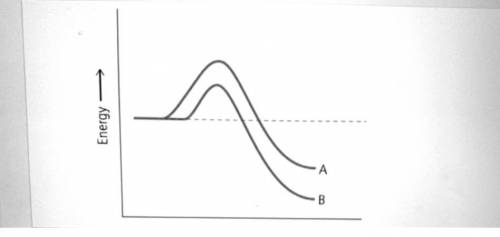
Reaction pathway A is exothermic. neither exothermic nor endothermic. endothermic. Reaction pathway B is exothermic. neither exothermic nor endothermic. endothermic. Which reaction pathway has the faster rate of reaction and why? Reaction pathway B has the faster rate because the products have less energy than the products of reaction pathway A. Reaction pathway A has the faster rate because the products have more energy than the products of reaction pathway B. Reaction pathway B has the faster rate because the activation energy is smaller than the activation energy of reaction pathway A. Reaction pathway A has the faster rate because the activation energy is greater than the activation energy of reaction pathway B. Which reaction pathway has the larger heat of reaction, Δ H rxn ? ΔHrxn?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
Reaction pathway A is exothermic. neither exothermic nor endothermic. endothermic. Reaction pathway...
Questions

Mathematics, 09.02.2022 08:10





Law, 09.02.2022 08:10










Mathematics, 09.02.2022 08:10

Mathematics, 09.02.2022 08:20