Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

You know the right answer?
A stock solution of HNO3HNO3 is prepared and found to contain 12.7 M of HNO3HNO3. If 25.0 mL of the...
Questions


History, 17.01.2020 02:31

Mathematics, 17.01.2020 02:31

















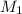 = 12.7 M,
= 12.7 M, 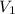 = 25.0 ml = 0.025 L (as 1 ml = 0.001 L)
= 25.0 ml = 0.025 L (as 1 ml = 0.001 L)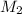 = ? ,
= ? , 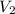 = 0.5 L
= 0.5 L