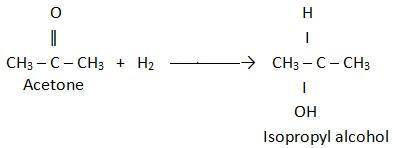
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon–oxygen double bond. calculate the enthalpy of reaction using the bond energies given. bond: c=o h–h c–h o–h c–c c–o bond energy (kj/mol): 745 432 413 467 347 358 multiple choice –61 kj +61 kj –366 kj –484 kj +366 kj

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon–oxygen do...
Questions


Mathematics, 23.05.2021 16:40

Biology, 23.05.2021 16:40


Chemistry, 23.05.2021 16:40


Biology, 23.05.2021 16:40

Mathematics, 23.05.2021 16:40

Biology, 23.05.2021 16:40


Mathematics, 23.05.2021 16:50


Mathematics, 23.05.2021 16:50


Mathematics, 23.05.2021 16:50




English, 23.05.2021 16:50

Social Studies, 23.05.2021 16:50