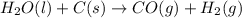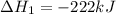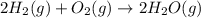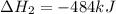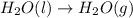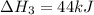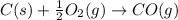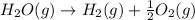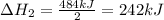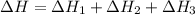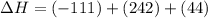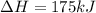Chemistry, 05.05.2020 16:29 shawn20034
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemical data above to calculate the change in enthalpy for the reaction below. H2O(l)+C(s)→CO(g)+H2(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemic...
Questions


Mathematics, 20.03.2020 18:27





History, 20.03.2020 18:28


History, 20.03.2020 18:28

Mathematics, 20.03.2020 18:28

Mathematics, 20.03.2020 18:28


English, 20.03.2020 18:29

Mathematics, 20.03.2020 18:29


SAT, 20.03.2020 18:29

English, 20.03.2020 18:30

Mathematics, 20.03.2020 18:30