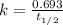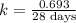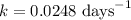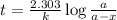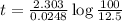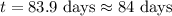Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
One of the radioactive isotopes used in medical treatment or analysis is chromium-51. The half-life...
Questions


Mathematics, 04.04.2020 11:09




Biology, 04.04.2020 11:09


History, 04.04.2020 11:09

Mathematics, 04.04.2020 11:09