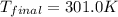Chemistry, 05.05.2020 15:04 BeautyxQueen
A 31.5 g wafer of pure gold initially at 69.4 ∘C is submerged into 63.4 g of water at 27.4 ∘C in an insulated container.
What is the final temperature of both substances at thermal equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

You know the right answer?
A 31.5 g wafer of pure gold initially at 69.4 ∘C is submerged into 63.4 g of water at 27.4 ∘C in an...
Questions

Biology, 22.04.2020 19:15

Advanced Placement (AP), 22.04.2020 19:15


Biology, 22.04.2020 19:15


Biology, 22.04.2020 19:15

Chemistry, 22.04.2020 19:15



Mathematics, 22.04.2020 19:16

Mathematics, 22.04.2020 19:16

Mathematics, 22.04.2020 19:16

Mathematics, 22.04.2020 19:16

Social Studies, 22.04.2020 19:16




Mathematics, 22.04.2020 19:16


Mathematics, 22.04.2020 19:16

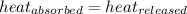
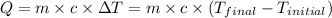
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0639/3665/09236.png) .................(1)
.................(1)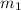 = mass of gold = 31.5 g
= mass of gold = 31.5 g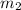 = mass of water = 63.4 g
= mass of water = 63.4 g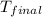 = final temperature = ?
= final temperature = ?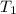 = temperature of gold =
= temperature of gold = 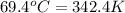
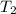 = temperature of water =
= temperature of water = 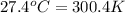
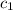 = specific heat of gold =
= specific heat of gold = 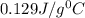
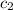 = specific heat of water=
= specific heat of water= 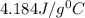
![-31.5\times 0.129\times (T_{final}-342.4)=[63.4\times 4.184\times (T_{final}-300.4)]](/tpl/images/0639/3665/eb953.png)