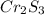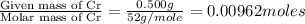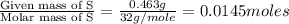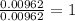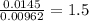Chemistry, 05.05.2020 15:08 teionamwhite2262
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate the empirical formula of the chromium sulfide.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3
You know the right answer?
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate...
Questions


History, 08.05.2021 02:40

Mathematics, 08.05.2021 02:40



Mathematics, 08.05.2021 02:40

Spanish, 08.05.2021 02:40

History, 08.05.2021 02:40




Computers and Technology, 08.05.2021 02:40


Mathematics, 08.05.2021 02:40


History, 08.05.2021 02:40

Mathematics, 08.05.2021 02:40


Mathematics, 08.05.2021 02:40

Mathematics, 08.05.2021 02:40