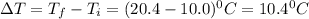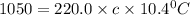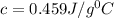Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
What is the specific heat of iron, if it requires 1050 J of heat energy to raise the temperature of...
Questions


Computers and Technology, 18.12.2020 09:10

Physics, 18.12.2020 09:10




History, 18.12.2020 09:10


Mathematics, 18.12.2020 09:10

Chemistry, 18.12.2020 09:10

Mathematics, 18.12.2020 09:10


Mathematics, 18.12.2020 09:10

History, 18.12.2020 09:10




Mathematics, 18.12.2020 09:10


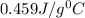
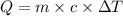
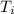 = 10.0°C
= 10.0°C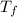 = 20.4°C
= 20.4°C