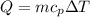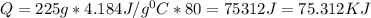Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 12:30
15) a substance used in manufacturing gasoline consists of finely divided platinum supported on an inert solid. suppose that the platinum is formed by the high temperature reaction between platinum (iv) oxide and hydrogen gas. the other product is water. a) write and balance the equation b) how many grams of hydrogen are needed to produce 1.0 g of platinum metal? c) how many moles of water are produced at the same time? how many grams? ( show work, .)
Answers: 1
You know the right answer?
A student must use 225 g of hot water in a lab procedure. Calculate the amount of heat in joules req...
Questions




Mathematics, 30.07.2019 09:30






Social Studies, 30.07.2019 09:30

Mathematics, 30.07.2019 09:30


English, 30.07.2019 09:30




Mathematics, 30.07.2019 09:30

Chemistry, 30.07.2019 09:30


Arts, 30.07.2019 09:30

 ) = 100°C - 20°C = 80°C, Water’s specific heat capacity (
) = 100°C - 20°C = 80°C, Water’s specific heat capacity (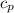 ) = 4.184 J/g°C
) = 4.184 J/g°C